Study reveals a key hormonal circuit in the kidneys Scripps Research scientists identify the protein that helps kidney cells regulate renin, providing foundational insight into how kidneys function.
December 19, 2025
LA JOLLA, CA The kidneys play a central role in keeping the body's internal environment stable. By filtering blood, removing waste and carefully controlling fluid and sodium levels, they help maintain healthy blood pressure while ensuring that organs receive the blood they need to function. To carry out this balancing act, the kidneys rely on hormones that respond to constant changes in the body, such as shifts in hydration and sodium intake. One of the most important of these hormones is renin. But how kidney cells sense fluctuations in physical forces like blood flow and pressure and translate those cues into precise control of renin release has remained unclear.
Now, scientists at Scripps Research and collaborating institutes have identified a mechanism within the kidneys that allows renin to adjust in real time. Published in Cell on December 4, 2025, the team's study shows that a protein called PIEZO2 enables kidney cells to gauge physical forces and alter renin output accordingly. The results add fundamental knowledge about how the kidneys maintain stability and what may go wrong in conditions marked by abnormal blood pressure or kidney performance.
Our findings emerged from a nearly six-year effort, and they explain the process by which the kidneys take a physical force and turn it into a cellular response, says co-corresponding author Professor Ardem Patapoutian, a Howard Hughes Medical Institute (HHMI) Investigator and the Presidential Endowed Chair in Neurobiology at Scripps Research. It's important for understanding how renin is regulated and what can happen when that control falters.
Patapoutian shared the 2021 Nobel Prize in Physiology or Medicine for discovering the cellular sensors that perceive touch and pressure. These sensors are ion channels protein gateways embedded in the cell membrane that open in response to physical force. The channel proteins PIEZO1 and PIEZO2 allow charged particles called ions to enter the cell, triggering signals that help coordinate vital functions throughout the body. For example, Patapoutian and a team of researchers recently demonstrated that PIEZO channels contribute to regulating uterine contractions during childbirth by sensing pressure and stretch.
Building on that foundation, this current study evaluated whether similar force-sensing mechanisms also regulate renin in the kidneys. The investigation accelerated when co-corresponding and first author Assistant Professor Rose Hill, a former HHMI Helen Hay Postdoctoral Fellow in Patapoutians laboratory, noticed an unexpected pattern while studying PIEZO channels in mouse kidney tissue.
I began the project through a bit of serendipity, recalls Hill, who now leads a research group at Oregon Health & Science University.
Initially, Hill had been examining PIEZO1 in the kidneys because it appears in many non-nerve tissues, including kidney cells. By contrast, PIEZO2 is usually linked to sensory nerve cells that detect light touch and body position so Hill wasn't expecting it to play a major role in kidney function.
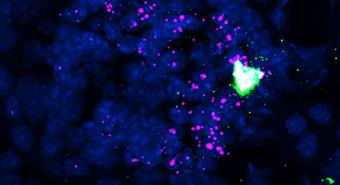
I didnt think PIEZO2 would be involved, but it wound up having this beautiful expression pattern in the juxtaglomerular granular cells the cell type responsible for producing renin, she elaborates.
Juxtaglomerular granular cells line the blood vessels that deliver blood into the kidneys' filters called the glomeruli where waste and excess fluid are removed from the bloodstream. These cells adjust renin levels based on chemical cues like sodium concentration, and past research suggested they may also respond to fluctuations in blood flow and pressure. However, scientists didn't know which protein was responsible for sensing those fluctuations.
This new study found that juxtaglomerular granular cells use PIEZO2 to detect such changes, producing calcium signals that act as internal messengers within the cells. Calcium signaling is a common way cells translate incoming cues into action, enabling activation or suppression of specific responses. In this case, the signals helped fine-tune renin release a process the team observed directly via live imaging of the kidneys.
To test whether PIEZO2 is required for normal kidney function, the team genetically removed the protein from renin-expressing cells in mice. Under typical conditions, these cells generated rhythmic calcium signals that pulsed in sync with the natural contraction and relaxation of blood vessels. When PIEZO2 was removed, the signals almost completely disappeared.
Because calcium signaling halts renin release, losing it meant the cells no longer received feedback to dial down renin. As a result, renin levels remained abnormally high even in circumstances where they would otherwise fall and the hormonal system struggled to adjust to changes in hydration and sodium intake.
Yet one of the most unexpected findings was how removing PIEZO2 affected kidney filtration. The team observed glomerular hyperfiltration when glomeruli filter blood too quickly similar to what's seen early in certain kidney disorders. But further analysis showed no evidence of underlying illness.
The hyperfiltration was largely driven by excess production of angiotensin-(1-7), a hormone that relaxes and widens blood vessels. When those vessels relax, more blood rushes into the glomeruli at once, causing filtration to speed up. When the researchers blocked the signal that causes vessel relaxation, filtration returned to normal.
Although the results are preclinical, they lay groundwork for future studies of human kidney physiology and disease. This insight could inform research on conditions marked b
Most recent headlines
05/01/2027
Worlds first 802.15.4ab-UWB chip verified by Calterah and Rohde & Schwarz to be ...
01/06/2026
January 6 2026, 05:30 (PST) Dolby Sets the New Standard for Premium Entertainment at CES 2026
Throughout the week, Dolby brings to life the latest innovatio...
02/05/2026
Dalet, a leading technology and service provider for media-rich organizations, t...
01/05/2026
January 5 2026, 18:30 (PST) NBCUniversal's Peacock to Be First Streamer to ...
01/04/2026
January 4 2026, 18:00 (PST) DOLBY AND DOUYIN EMPOWER THE NEXT GENERATON OF CREATORS WITH DOLBY VISION
Douyin Users Can Now Create And Share Videos With Stun...
12/02/2026
Share
Copy link
Facebook
X
Linkedin
Bluesky
Email...
12/02/2026
Share
Copy link
Facebook
X
Linkedin
Bluesky
Email...
12/02/2026
Share
Copy link
Facebook
X
Linkedin
Bluesky
Email...
12/02/2026
Share
Copy link
Facebook
X
Linkedin
Bluesky
Email...
12/02/2026
Share
Copy link
Facebook
X
Linkedin
Bluesky
Email...
12/02/2026
TIME100 Health list features Scripps Research Professor Darrell Irvine Irvine is recognized for his work in empowering the immune system to fight disease, which...
11/02/2026
FYI: Phone Support Maintenance One thing we pride ourselves on here at Utah Scientific is our 24-hour support included with our signature 10-year hardware warra...
11/02/2026
Leading provider of video streaming solutions, Bitmovin, has appointed Ian Baglow as Co-CEO alongside existing CEO and Co-Founder Stefan Lederer. Under this str...
11/02/2026
Paramount and the CBS Television Network will partner to air UFC 326: HOLLOWAY vs. OLIVEIRA 2 live on Saturday, March 7, from T-Mobile Arena in Las Vegas, mar...
11/02/2026
Beginning February 10, fans can buy MLB.TV on ESPN, a new milestone in one of sports media's longest-standing partnerships. ESPN becomes the new streaming h...
11/02/2026
Fubo Sports Network is available to Hulu's Live TV subscribers in the core $89.99 a month subscription plan, which also includes full access to the entire H...
11/02/2026
Following a competitive public tender process, Rai (Radiotelevisione Italiana), the national public broadcasting company of Italy, has awarded Imagine Communica...
11/02/2026
Major League Baseball is making in-market streaming subscriptions for 20 Clubs available today for fans. Subscriptions for the following Clubs are available vi...
11/02/2026
Building on successful demonstrations during the Paris Olympics 2024, Italian public service broadcaster Rai and the European Broadcasting Union (EBU) are condu...
11/02/2026
Following Sunday's Super Bowl LX, ESPN and Disney unveiled We're Going,...
11/02/2026
Delayed streams are a growing source of frustration for sports fans. During the 2026 Super Bowl, some streams lagged up to 62 seconds behind the action on the f...
11/02/2026
NASCAR and FloSports announces an expanded slate of racing events that will bring FloRacing coverage live throughout the 2026 season to the NASCAR Channel, furt...
11/02/2026
Manifold technologies GmbH announces the appointment of Nick Tucker as Sales Manager for Europe, reinforcing the company's continued growth across broadcast...
11/02/2026
Genies, the AI avatar technology company powering the next era of interactive digital identity, entered into a landmark collaboration with MLB Players, Inc., th...
11/02/2026
The International Cricket Council (ICC) and Google have joined forces for an AI-...
11/02/2026
Dolby's CEO Kevin Yeaman and Giles Baker, SVP of Dolby Cloud Solutions, shared how the brand's latest innovations - Dolby Vision, Dolby Atmos, and Dolby...
11/02/2026
Ilitch Sports + Entertainment has entered a first of its kind partnership with Major League Baseball, which will provide broadcast support to both the Detroit T...
11/02/2026
For major U.S. events like Super Bowl 2026, FIFA World Cup, America 250, and the...
11/02/2026
Broadcasts of the NHL's Detroit Red Wings will also be produced by the leagu...
11/02/2026
Video moves fast can your DAM keep up?
Join Blue Lucy in LA for the West Coast's leading Digital Asset Management event as we explore, celebrate, and acc...
11/02/2026
NEW YORK - February 10, 2026 - An estimated 124.9 million viewers watched Super Bowl LX on Sunday, February 8, according to Nielsen's Big Data Panel measu...
11/02/2026
Share
Copy link
Facebook
X
Linkedin
Bluesky
Email...
11/02/2026
Share
Copy link
Facebook
X
Linkedin
Bluesky
Email...
11/02/2026
Share
Copy link
Facebook
X
Linkedin
Bluesky
Email...
11/02/2026
Clear-Com provided an advanced, IP-based communications infrastructure for TEDNext 2025, supporting production, media, and editorial teams with a highly flexib...
11/02/2026
Astera introduces QuikBeam, the newest addition to its acclaimed Quik family of focusing LED Fresnels. This ultra-compact spotlight combines the equivalent powe...
11/02/2026
Following a competitive public tender process, Rai (Radiotelevisione Italiana), the national public broadcasting company of Italy, has awarded Imagine Communica...
11/02/2026
With Convertible Mount for NL Bowens & Aputure A Mounts See it at BSC Expo Stand #133 LCA
DoPchoice continues to refine light shaping tools for professional LE...
11/02/2026
World Premiere at BSC Expo, Booth #319 Oberkochen/Germany, 10 February 2026
ZEISS introduces the new Aatma, set of nine high-end full frame T1.5 cinema primes ...
11/02/2026
As Re-recording Mixer and Head of Sound at The Farm, one of UK's leading post-production facilities, Nick Fry has built his career on making stories sound a...
11/02/2026
Share
Copy link
Facebook
X
Linkedin
Bluesky
Email...
11/02/2026
Share
Copy link
Facebook
X
Linkedin
Bluesky
Email...
11/02/2026
Share
Copy link
Facebook
X
Linkedin
Bluesky
Email...
11/02/2026
Share
Copy link
Facebook
X
Linkedin
Bluesky
Email...
11/02/2026
Graduate Spotlight: Gabrielle Rodriguez The educator, who grew up in the Philippines, shares how shes bringing what she learned at Berklee back home.
Februar...
11/02/2026
Wednesday 11 February 2026
Sky brings together Netflix, Disney , HBO Max and Ha...
11/02/2026
Back to All News
Netflix Confirms Production of Love O'Clock' From the...
11/02/2026
Back to All News
Investing in Belgian Stories: A Commitment to Culture and Choice
From left to right: Undercover, Ang le, Rough Diamonds, Into the Night, John...
11/02/2026
At the end of January, ICG headed off to the Portuguese capital, Lisbon, for our annual conference.
An early flight gave us plenty of time to start exploring s...
11/02/2026
ABS Strengthens Ku-Band Capacity and Expands Regional Reach Through Strategic Pa...
 Study reveals a key hormonal circuit in the kidneys Scripps Research scientists identify the protein that helps kidney cells regulate renin, providing foundational insight into how kidneys function.
Study reveals a key hormonal circuit in the kidneys Scripps Research scientists identify the protein that helps kidney cells regulate renin, providing foundational insight into how kidneys function.











































